20/09/2023
Neurological conditions can have a substantial impact on one’s quality of life, and on those who care for them. It’s essential to identify these conditions early, as it allows for more effective management and treatment. Early diagnosis can lead to better health outcomes, reduced complications, and even lower treatment costs.
Particularly in the case of neurodegenerative diseases like Alzheimer’s and Parkinson’s, the importance of early diagnosis cannot be understated. These diseases progress over time, causing irreversible damage to the brain. Detecting these conditions early offers the advantage of time to plan, make informed decisions about care, and access appropriate treatments.
As part of the Signature Health Check, several important neurological biomarkers are tested to provide data about your neurological health. Let’s explore some of these biomarkers and understand their role in brain health.
Neurological Biomarkers
D-dimer is a small protein fragment produced during the breakdown of blood clots. This means D-dimer is a useful marker for the presence of blood clots, which if present in the blood vessels that supply the brain, can increase the risk of stroke. D-dimer has been shown to be a good predictor of stroke outcomes, with a hazard ratio of 2.291. In other words, high levels of plasma D-dimer are associated with a 2.29x increased likelihood of post-stroke mortality when compared with those who display low levels of D-dimer.
In addition to classic stroke, D-dimer has also been shown to be elevated in cerebral venous thrombosis (CVT), a rare type of stroke that occurs due to a blood clot in the veins that drain blood from the brain. Increased levels of D-dimer have been shown to indicate an increased risk of focal neurological deficits in CVT. Focal neurological deficits refer to the symptoms that occur when a specific area of the brain is damaged or not functioning properly2. These symptoms can include weakness or paralysis on one side of the body, difficulty speaking or understanding language, vision problems, and sensory changes, however, the specific symptoms depend on which veins are affected and the extent of the blockage. Finally, D-dimer has been shown to be elevated in response to head injuries and is a useful predictor of neurological function prognosis3.
Finally, D-Dimer has been shown to be elevated in response to head injuries and is a useful predictor of neurological function prognosis3.
Glial fibrillary acidic protein (GFAP) is primarily expressed by astrocytes, a type of glial cell found in the central nervous system (CNS) that provides structural and metabolic support to neurons4. Like many other neurological markers, the exact function of this protein is not fully understood. Nonetheless, it is associated with a few neurological conditions. High levels of GFAP in the blood can arise from brain injury resulting from head trauma or stroke. It is a particularly useful test for differentiating between different types of stroke, with higher levels observed in haemorrhagic stroke (stroke caused by a brain bleed) than ischaemic stroke (stroke caused by a blood clot). In addition, GFAP levels have been shown to correlate with the severity of spinal cord injury, providing an early indication of how bad the injury is. GFAP is also associated with a variety of neurodegenerative conditions, including Alzheimer’s disease. Studies show that levels of GFAP are elevated in those who suffer from Alzheimer’s disease5.
Other conditions associated with GFAP include Neuromyelitis Optica Spectrum Disorders (NMSOD). NMOSD is a group of rare, autoimmune conditions that primarily target the optic nerves and spinal cord, leading to inflammation and damage to these areas. GFAP levels in NMSOD patients tend to be higher than those seen in those who suffer from multiple sclerosis, a disease commonly mistaken for NMSOD6.
Glutathione S-transferase Pi is an enzyme involved in the detoxification of many compounds. This includes combating oxidative stress. Oxidative stress is an imbalance in the body’s production of reactive oxygen species and your ability to neutralise them with antioxidants and is thought to be a driver for the development and progression of Alzheimer’s disease7.
Interleukin-6 (IL-6) is a cytokine involved in various physiological and pathological processes in the CNS. It is associated with neuroinflammation as seen in conditions such as systemic lupus and bacterial meningitis8, neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease9, neuroprotection10, and other conditions such as multiple sclerosis11. The neuroprotective effects of IL-6 are displayed by its ability to improve infarct volumes (a measure of the damage caused by a stroke) and neurological scores, reduce neuronal death and improve the integrity of the blood-brain barrier10
Nucleoside diphosphate kinase A (NDKA) is an enzyme with roles in various cellular functions including nucleotide metabolism and signalling pathways. NDKA has been implicated as a potential marker for the early diagnosis of stroke12.
PARK7, also known as DJ-1, is a gene that encodes a protein with several functions, including the protection of cells, particularly brain cells, from oxidative stress. PARK7 was originally identified as an oncogene upregulated in different types of cancer, it has also been implicated as a causative factor for neurodegeneration in rare inherited forms of Parkinson’s disease. Mutations in the PARK7 gene can prevent the production of any functional PARK7 protein, which may lead to Parkinson’s disease13. PARK7 protein has also been shown to be a strong, early predictor of stroke12.
Neurological Conditions
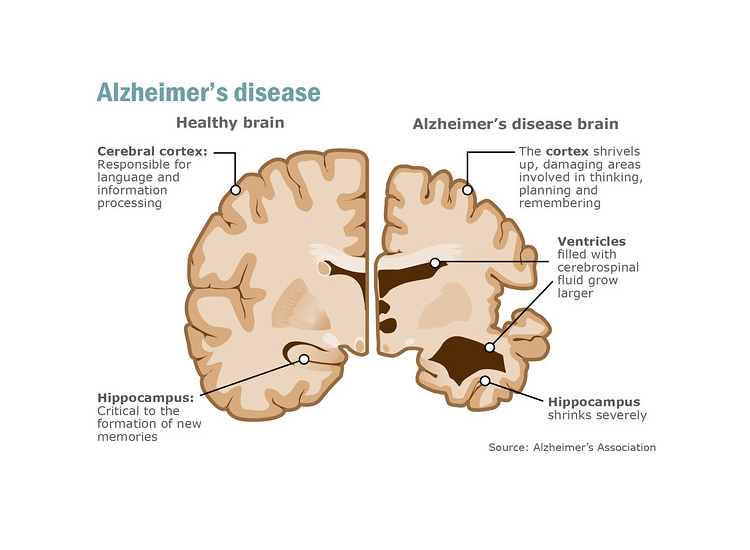
Alzheimer’s disease is a neurodegenerative disorder that primarily affects memory, thinking, and behaviour. There is currently no cure for Alzheimer’s disease, there are treatments available that can help manage symptoms and improve quality of life. These treatments are more effective the earlier the condition is diagnosed.
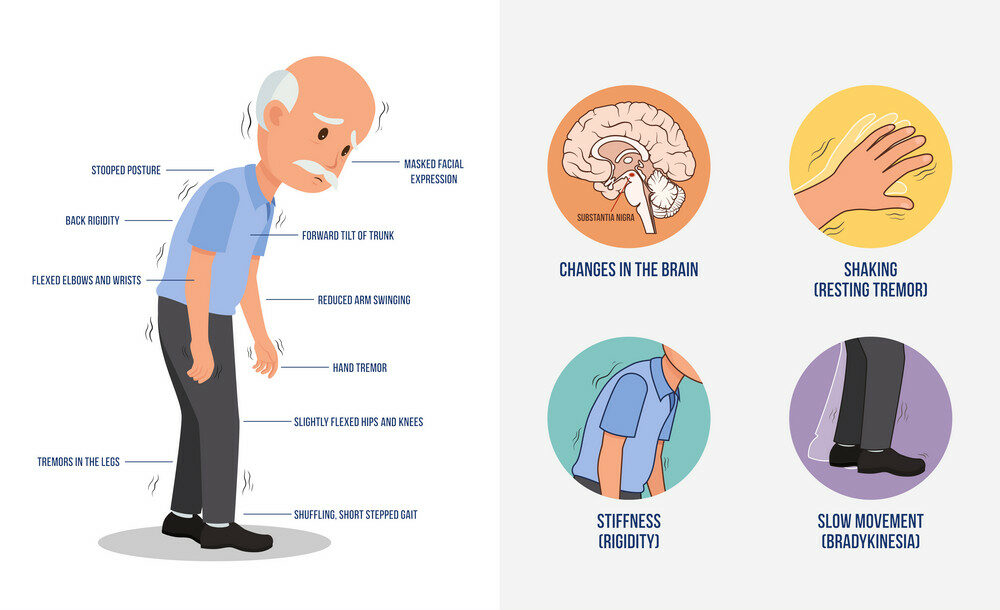
Parkinson’s disease is a progressive neurological condition that affects parts of the brain. It is a neurodegenerative disorder that affects predominately the dopamine-producing neurons in a specific area of the brain called the substantia nigra. Symptoms usually begin gradually and worsen over time, and may include tremors, stiffness, slow movement, difficulty with balance and coordination, depression, anxiety, and memory difficulties. There is currently no cure for Parkinson’s disease, but treatments are available that can help manage symptoms and improve quality of life. Like Alzheimer’s disease, the earlier Parkinson’s disease is identified, the more favourable the outlook.
Conclusion
The Randox Health Signature health programmes incorporate testing of six key neurological biomarkers to provide an analysis of your neurological health. This not only provides insights into the possible progression of various neurodegenerative and neurological conditions but also underscores the importance of early detection. Recognising these conditions early can significantly improve management and treatment strategies, positively influence prognosis, and reduce associated healthcare costs. Often, by the time symptoms appear, it’s too late to effectively halt the progression of these conditions. Proactive testing is crucial as it can greatly enhance the health outcomes related to neurological conditions.
Reference List
- Zhang P, Wang C, Wu J, Zhang S. A Systematic Review of the Predictive Value of Plasma D-Dimer Levels for Predicting Stroke Outcome. Front Neurol. 2021;12. doi:10.3389/fneur.2021.693524
- Juli C, Amalia L, Gamayani U, Atik N. D-Dimer Level Associates with the Incidence of Focal Neurological Deficits in Cerebral Venous Thrombosis Patients. J Blood Med. 2020;Volume 11:449-455. doi:10.2147/JBM.S283633
- Asami M, Nakahara S, Miyake Y, et al. Serum D-dimer level as a predictor of neurological functional prognosis in cases of head injuries caused by road traffic accidents. BMC Emerg Med. 2022;22(1):51. doi:10.1186/s12873-022-00613-9
- Žurek J. Biomarkers in Traumatic Brain Injury. In: Essentials of Neuroanesthesia. Elsevier; 2017:587-591. doi:10.1016/B978-0-12-805299-0.00033-6
- Leuzy A, Mattsson‐Carlgren N, Palmqvist S, Janelidze S, Dage JL, Hansson O. Blood‐based biomarkers for Alzheimer’s disease. EMBO Mol Med. 2022;14(1). doi:10.15252/emmm.202114408
- Kim H, Lee EJ, Lim YM, Kim KK. Glial Fibrillary Acidic Protein in Blood as a Disease Biomarker of Neuromyelitis Optica Spectrum Disorders. Front Neurol. 2022;13. doi:10.3389/fneur.2022.865730
- Parvizi T, König T, Wurm R, et al. Real-world applicability of glial fibrillary acidic protein and neurofilament light chain in Alzheimer’s disease. Front Aging Neurosci. 2022;14. doi:10.3389/fnagi.2022.887498
- Gruol DL, Nelson TE. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol Neurobiol. 1997;15(3):307-339. doi:10.1007/BF02740665
- Rothaug M, Becker-Pauly C, Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research. 2016;1863(6):1218-1227. doi:10.1016/j.bbamcr.2016.03.018
- Kummer KK, Zeidler M, Kalpachidou T, Kress M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine. 2021;144:155582. doi:10.1016/j.cyto.2021.155582
- Stampanoni Bassi M, Iezzi E, Drulovic J, et al. IL-6 in the Cerebrospinal Fluid Signals Disease Activity in Multiple Sclerosis. Front Cell Neurosci. 2020;14. doi:10.3389/fncel.2020.00120
- Allard L, Burkhard PR, Lescuyer P, et al. PARK7 and Nucleoside Diphosphate Kinase A as Plasma Markers for the Early Diagnosis of Stroke. Clin Chem. 2005;51(11):2043-2051. doi:10.1373/clinchem.2005.053942
- Mencke P, Boussaad I, Romano CD, Kitami T, Linster CL, Krüger R. The Role of DJ-1 in Cellular Metabolism and Pathophysiological Implications for Parkinson’s Disease. Cells. 2021;10(2):347. doi:10.3390/cells10020347



