13/09/2023
In the quest to conquer cancer, one of the most promising advancements is the rise of genetic testing. These revolutionary tools allow us to peek into our DNA, identify potential threats and enable us to take proactive steps towards prevention and early detection. At Randox Health, we’re leading the charge in providing personalised health insights through innovative testing methods.
The Genetic Cancer Risk test stands out for its potential to transform the landscape of cancer prevention and early risk detection. By leveraging the capabilities of genetic testing, this service delivers vital health information to individuals, empowering them to take a proactive stance in managing their health
What is Genetic Testing
Genetic testing identifies changes, or mutations, in a person’s genes that could increase their risk of developing a disease, in this case, cancer. These mutations can be inherited from our parents or can occur spontaneously1. Some of the most important genetic alterations associated with cancer include mutations in the BRCA1 and BRCA2 genes, which are associated with an increased risk of breast and ovarian cancer, and mutations in genes like TP53 and MLH1, which are linked to various forms of cancer2. We’ll look at these in a bit more detail later.
These genetic alterations can lead to the development of cancer by causing cells to grow and divide uncontrollably, leading to the formation of a tumour. Understanding these genetic changes can help doctors predict a person’s risk of developing certain types of cancer and guide treatment decisions if cancer does develop1.
Genetic testing for cancer risk typically involves a blood or saliva sample. The sample is sent to a laboratory where it is analysed for mutations in specific genes associated with an increased risk of cancer. The results of these tests can provide valuable information about an individual’s cancer risk and guide decisions about prevention and early detection strategies1.
It’s important to note that a positive test result does not necessarily mean a person will develop cancer, but rather that they are at higher risk. Conversely, a negative result does not guarantee that a person will not get cancer, as many cancers are not caused by inherited mutations but by mutations that occur over a person’s lifetime1.
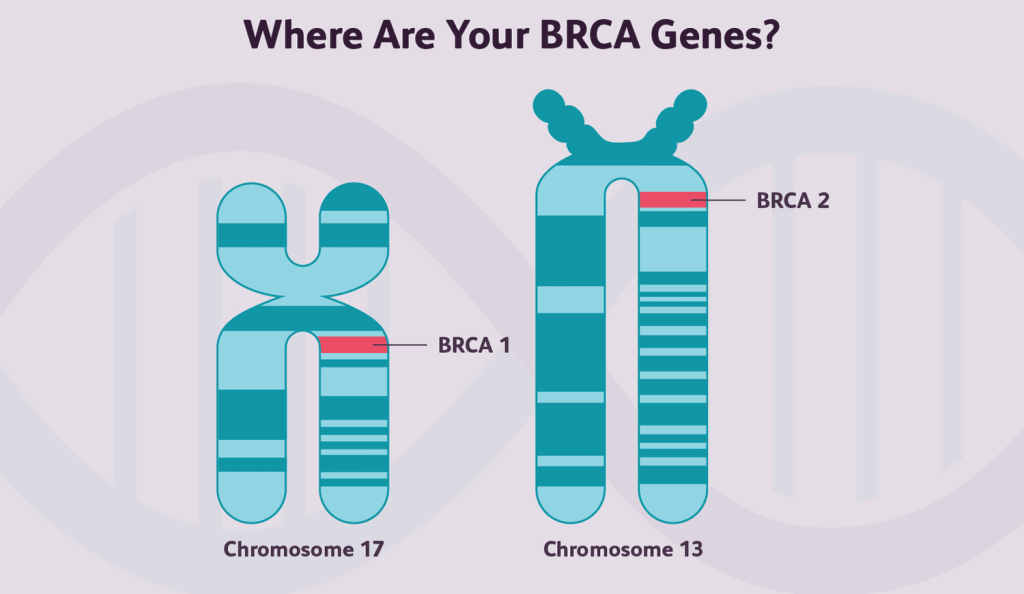
BRCA1 and BRCA2 Gene
BRCA1 and BRCA2 genes are two of the most well-studied genes in the context of cancer risk. Mutations in these genes are associated with an increased risk of breast and ovarian cancer. The BRCA1 and BRCA2 genes are tumour suppressors that play a critical role in DNA repair. When these genes contain harmful variants, the DNA repair process can be compromised, leading to increased cancer risk2. Genetic testing for BRCA1 and BRCA2 mutations is now a standard component of risk assessment for breast and ovarian cancer. Individuals who carry these mutations may choose to undergo increased surveillance or surgery to manage their cancer risk.
TP53
The TP53 gene is one of the most frequently mutated genes in human cancers. Mutations in TP53 can lead to the production of a dysfunctional p53 protein, allowing cells with damaged DNA to continue to divide and potentially form a tumour3. Genetic testing for TP53 mutations can identify individuals with increased risk and guide strategies for cancer prevention and early detection.
MLH1
The MLH1 gene helps to correct errors that occur during DNA replication. Mutations in MLH1 can lead to a condition known as Lynch syndrome, which significantly increases the risk of colorectal cancer, endometrial cancer, and several other types of cancer4. Genetic testing for MLH1 mutations can identify individuals with Lynch syndrome and guide strategies for cancer prevention and early detection. For example, individuals with Lynch syndrome may benefit from regular colonoscopies starting at an earlier age than the general population.
The Role of Genetic Counselling
Given the complexities and potential implications of genetic testing, genetic counselling is a crucial part of the process. Genetic counsellors are specialised healthcare professionals who possess the expertise to guide individuals through the complex decision-making process for genetic testing5.
They begin with a comprehensive evaluation of an individual’s personal and family medical history to assess the potential risk of genetic disorders. They are skilled in identifying patterns that may suggest a hereditary condition and can help determine whether genetic testing may be beneficial5.
Once a decision to proceed with genetic testing is made, genetic counsellors provide critical support in interpreting the results. They translate genetic data into understandable terms, explaining what the results mean for the individual’s health, the risk of developing a certain condition, and the potential implications for family members5.
Furthermore, they discuss potential management strategies based on the genetic test results, which can range from enhanced screening and surveillance to preventive measures or targeted therapies6.
In essence, genetic counselling is a patient-centred service that empowers individuals with the knowledge to make informed decisions about their health, ensuring they fully understand and can effectively manage their genetic health risks.

Genetic Cancer Risk
The Genetic Cancer Risk test covers 94 genes suspected to play a role in predisposition to cancer, including genes associated with increased risk of breast, ovarian, prostate, colorectal, thyroid cancer and more. This test is designed to provide a more comprehensive analysis of a person’s genetic risk for developing cancer. The test includes a consultation with a genetic counsellor, DNA analysis performed on a simple blood sample, and results within six weeks.
View the list of genes tested:
AIP, ALK, APC, ATM, BAP1, BMPR1A, BLM, BRCA1, BRCA2, BRIP1, BUB1B, CDH1, CDK4, CDKN1C, CDKN2A, CEBPA, CEP57, CHEK2, CYLD, DDB2, DICER1, DIS3L2, EGFR, EPCAM, ERCC2, ERCC3, ERCC4, ERCC5, EZH2, EXT1, EXT2, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, FH, FLCN, GATA2, GPC3, HRAS, HNF1A, KIT, MAX, MEN1, MET, MLH1, MUTYH, NBN, NF1, NF2, NSD1, PALB2, PHOX2B, PMS1, PMS2, PRF1, PRKAR1A, PTCH1, PTEN, RAD51C, RAD51D, RECQL4, RET, RHBDF2, RUNX1, SBDS, SDHAf2 , SDHB , SDHC , SDHD , SLX4 , SMAD4 , SMARCB1 , STK11 , SUFU , TSC1 , TSC2 , TMEM127 , VHL , WRN , XPA , XPC
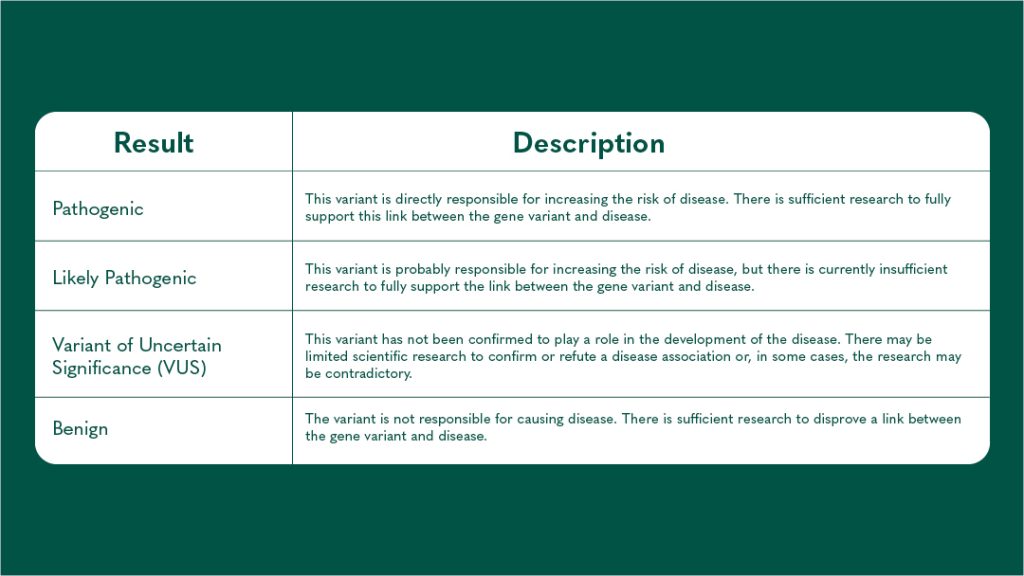
Results of your Genetic Testing
When you receive the results from your genetic testing, there are three possible results you might get. The table below details these:
In the fight against cancer, knowledge is power. Randox Health’s Genetic Cancer Panels provide valuable insights into your personal risk, empowering you to take control of your health. With these tools, we can move from a reactive approach to a proactive one, catching cancer before it starts or in its early stages when it’s most treatable. It’s not just about adding years to your life but adding life to your years.
References
- National Cancer Institute. Genetic Testing for Hereditary Cancer Syndromes. National Cancer Institute. Published 2012. https://www.cancer.gov/about-cancer/causes-prevention/genetics/genetic-testing-fact-sheet
- National Cancer Institute. BRCA Mutations: Cancer Risk & Genetic Testing. National Cancer Institute. Published November 19, 2020. https://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet
- Sorrell AD, Espenschied CR, Culver JO, Weitzel JN. TP53 Testing and Li-Fraumeni Syndrome: Current Status of Clinical Applications and Future Directions. Molecular diagnosis & therapy. 2013;17(1):31-47. doi:https://doi.org/10.1007/s40291-013-0020-0
- Dedeurwaerdere F, Claes KB, Van Dorpe J, et al. Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Scientific Reports. 2021;11(1):12880. doi:https://doi.org/10.1038/s41598-021-91974-x
- Nelson HD, Huffman LH, Fu R, Harris EL. Genetic Risk Assessment and BRCA Mutation Testing for Breast and Ovarian Cancer Susceptibility: Systematic Evidence Review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2005;143(5):362. doi:https://doi.org/10.7326/0003-4819-143-5-200509060-00012
- Patterson C, Feightner JW, Garcia A, Hsiung GY . R, MacKnight C, Sadovnick AD. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. Canadian Medical Association Journal. 2008;178(5):548-556. doi:https://doi.org/10.1503/cmaj.070796



